how many valence electrons does iron have|Iron Valence Electrons : Cebu The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated . Bet on Basketball with PokerStars Sports, including online NBA, Euroleague Men, and Eurocup Men betting odds and all other major Basketball betting markets
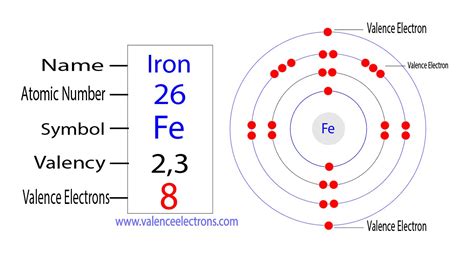
how many valence electrons does iron have,Find out the valence electrons of any element in the periodic table with this interactive chart. For iron (Fe), the valence electrons are 8, which means it belongs to the third period and group.Iron has 8 valence electrons, according to the answer from Socratic, a website for chemistry questions and answers. The answer explains the concept of valence electrons for .Find the valence of iron and other elements in this table based on the bond valence model. Iron has a valence of +2, +3, or +4 depending on the bond type.The total number of valence electrons for iron is 8: 2 electrons in the highest occupied energy level (n=4) plus 6 electrons in the (n-1) d orbital, that is, 3d. Using abbreviated .Iron Valence Electrons The valence electrons of iron are eight, determined by adding the total electrons of the d-orbital to the last shell. The valence electrons of iron ions are fourteen, determined by .
The electron configuration of iron is [Ar] 3d 6 4s 2, if the electron arrangement is through orbitals. The number of electrons in iron is 26 and the valence electrons are 3. Learn .
Learn how to determine the number of valence electrons for an element using the periodic table. Iron is in group 8 and has six valence electrons. See examples, diagrams, and tips .To find the number of valence electrons for Fe (Iron) we need to look at its electron configuration. This is necessary because Fe is a transition metal (d b.Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; aluminum; Solution. Sulfur .March 11, 2023. What are iron valence electrons? Iron valence electrons and their importance for chemistry. Different types of electron configurations can occur in iron .The electrons which are distributed in the outermost shell of the atom are called valence electrons. These valence electrons can form a chemical bond only if the outer shell remains unclosed. Valence electrons in an Iron(Fe atom: The element Iron Fe is in the fourth period and belongs to the d-block (group 8). The atomic number of Iron Fe is 26 .how many valence electrons does iron have Iron Valence Electrons Valence shell electrons (or, more simply, the valence electrons) are the electrons in the highest-numbered shell, or valence shell, while core electrons are the electrons in lower-numbered shells. We can see from the electron configuration of a carbon atom—1 s2 2 s2 2 p2 —that it has 4 valence electrons (2 s2 2 p2) and 2 core .
When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most . And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system .
Iron has 8 valence electrons. Explanation: Iron has an electron configuration $$\displaystyle{1}{s}^{2}{2}{s}^{2}{2}{p}^{6}{3}{s}^{2}{3}{p}^{6}{4}{s}^{2}{3}{d}^{6}$$ or $$[Ar]4s^23d%6$$. The electrons outside the noble gas core are? Right! The $$4s^23d^6$$ electrons. Iron thus has 8 valence electrons!
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . How many valence electrons does boron have? Recognize that the second principal energy level consists of both the \(2s\) and the \(2p\) sublevels, and so the answer is three. In fact, the number of valence electrons goes up by one for each step across a period, until the last element is reached. Neon, with its configuration ending in .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .

8. How many electrons does iron have? Iron contains eight electrons in its valence shell. Because iron is a transition metal, electrons in its d subshells can be used as valence electrons. In a transition metal, valence electrons are electrons that exist outside of a noble-gas core. Iron has the electrical configuration 1s 2 2s 2 2p 6 3s . For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Alkaline earth atoms (e.g., magnesium, calcium) have two valence electrons. The noble gases have complete octets, so all eight of their electrons are valence electrons. The exception is helium, which has two valence electrons. . The electron .

Write the complete electron configuration for element 114. How many valence electrons are found in the ground state electron configuration for Element 114? Answer: . How many unpaired electrons does iodine . How many valence electrons does iron have? Oxide ions tend to have a 2- charge. The overall charge of Fe3O4 is 3(Fe) + 4(-2) = 0, which means that the valency of iron in Fe3O4 is +8/3. But since . Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable . 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.how many valence electrons does iron have Iron (Fe) is a transition metal located in group 8 and period 4 of the periodic table. It has the following key properties related to its valence electrons: Atomic number of iron = 26. Electron configuration = 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Iron has 2 electrons in its outermost 4s orbital and 6 electrons in the 3d orbital.The element Iron was discovered by Unknown in year Before 5000 BCE . Iron was first isolated by Egypt in 4000 BCE. How many valence electrons does a Iron atom have? Iron has 3 valence electrons. Iron has 26 electrons out of which 3 valence electrons are present in the 3d6 4s2 outer orbitals of atom. What is the melting Point of Iron?View this answer. Iron has eight valence electrons. The quantity of valence electrons an element has can be determined by counting the number of electrons outside of. See full answer below.Atoms tend to have all its valence orbitals occupied by paired electrons. For transition metals, the valence orbitals consist of ns, 3 np and 5 (n-1)d orbitals, leading to its tendency of being surrounded by 18 electrons. This is somewhat analogous to the octet and Lewis structure rules of main group elements in a simplified rationale.
how many valence electrons does iron have|Iron Valence Electrons
PH0 · Valences of the Elements Chemistry Table
PH1 · Valence Electrons for Fe (Iron)
PH2 · Valence Electrons Chart for All Elements
PH3 · Iron Valence Electrons
PH4 · How to Find the Valence Electrons for Iron (Fe)?
PH5 · How many valence electrons does iron have? + Example
PH6 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH7 · Determine valence electrons using the periodic table
PH8 · 3.10: Valence Electrons
PH9 · 10.6: Valence Electrons